Abstract
Background:
Ponatinib is a potent oral tyrosine kinase inhibitor (TKI) approved for pts with CP-CML or Ph+ ALL for whom no other TKI therapy is indicated, or for pts with the BCR-ABL1 T315I mutation. The pivotal phase 2 ponatinib PACE trial (NCT01207440) enrolled pts with CML or Ph+ ALL resistant/intolerant to dasatinib or nilotinib, or with T315I. An accumulation of AOEs was observed with continued follow-up of patients in PACE, and in Oct '13, dose reductions were instructed to mitigate the risk of AOEs.
Aims:
Long-term follow-up (median of approximately 5 years) of the incidence and clinical characteristics of AOEs observed in CP-CML pts treated with ponatinib in PACE.
Methods:
The starting dose of ponatinib in PACE was 45 mg/day; protocol-defined dose modifications were in place for management of AEs. The primary endpoint in CP-CML was MCyR by 12 mos. Safety monitoring included collection of treatment-emergent AOEs (categorized based on a collection of >300 MedDRA preferred terms related to vascular ischemia or thrombosis), including: incidence, time to onset, dose at onset, and dose modifications due to AOEs. Exposure-adjusted incidence of new AOEs was calculated as the number of pts with events/100 pt-yrs. Final data as of 6 Feb '17 are reported, with a median follow-up of 56.8 mos in CP-CML pts.
Results:
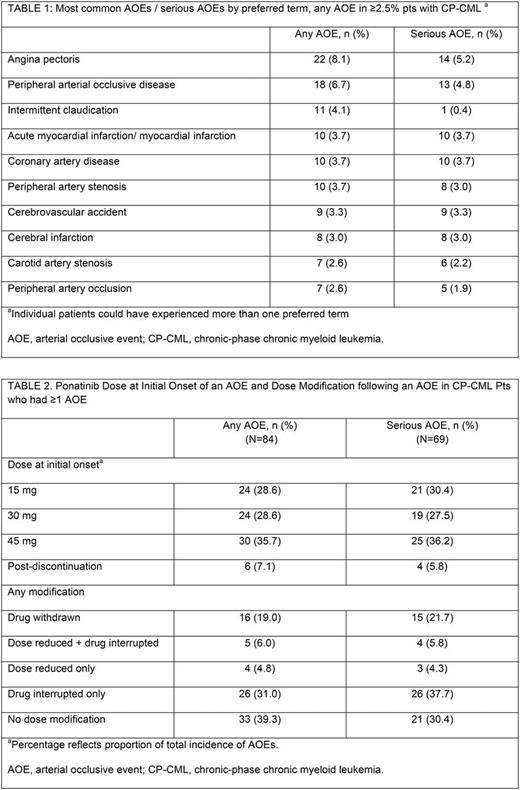
Of 270 CP-CML pts enrolled and treated in PACE, median age at baseline was 60 (18-94) yrs; median time from diagnosis to first dose was 7 (0.5-27) yrs; 60% received ≥3 prior TKIs. In efficacy evaluable pts (n=267), cumulative response rates as of data cutoff were: MCyR, 60%; CCyR, 54%; MMR, 40%; and MR4.5, 24%; the 5-yr OS probability was 77%. The cumulative incidence of any AOE/serious AOE in CP-CML pts, regardless of investigator attribution of causality, was 31%/26% (by subtype, 16%/12%, 13%/10%, and 14/11% for CV, cerebrovascular, and peripheral vascular AOEs, respectively). Most common AOEs/serious AOEs are reported in Table 1. The median time to onset for first CV, cerebrovascular, and peripheral vascular AOEs was 14.2 (range: 0.5-59.7), 22.8 (0.4-53.5) and 20.5 (0.3-58.5) mos, respectively. Among pts who experienced ≥1 AOE, ponatinib dose at initial onset of any AOE/serious AOE was most commonly at 45 mg/day (36%/36%, Table 2; by subtype, 38%/39% for CV AOEs, 29%/36% for cerebrovascular AOEs, and 24%/19% for peripheral vascular AOEs). When considering baseline risk factors for the development of serious AOEs, pts with >2 risk factors, including history of ischemic disease, had a higher relative risk of serious AOEs (2.9 [95% CI 1.8-4.9]), as compared to pts with 1 or no risk factors. Among pts who experienced ≥1 AOE, most (61%) were managed with dose modification, including reduction and/or interruption or withdrawal; 39% had no dose modification following the AOE (Table 2). Of 86 pts without a history of AOEs who had a dose reduction in response to the Oct '13 instructions, 12 (14%) had a first AOE after Oct '13 (among pts who dose reduced from 45 mg, 5 [11%] experienced a first AOE), compared to 8/59 (14%) of those who did not have a dose reduction in response to the Oct '13 instructions (1 [14%] pt receiving 45 mg). While the cumulative incidence of AOEs has increased over time in PACE, exposure-adjusted incidence of new AOEs has shown some decline over time (yr 1, 15.8; yr 2, 15.6; yr 3, 13.4; yr 4, 9.8; yr 5, 4.9). The ponatinib median dose intensities in these pts by yr were 32.1 mg/d, 31.4 mg/d, 24.8 mg/d, 19.0 mg/d, 20.4 mg/d, respectively. Grade 5 AOEs were reported in 3 CP-CML pts (1%): myocardial infarction, cerebrovascular accident and hemorrhagic cerebral infarction.
Summary:
Final (5-yr) results from PACE demonstrate that ponatinib continues to provide efficacy benefit to CP-CML pts. While the cumulative incidence of AOEs increased over time, the exposure-adjusted incidence of newly occurring AOEs seemed to trend down after the 3rd year; the lower exposure-adjusted AOE incidence in later years could be due to the natural etiology of AOEs, dose modifications, or change in the patient population. The most commonly reported AOE was angina pectoris and most AOEs were managed with dose modification. While the mechanistic basis for ponatinib-associated AOEs is unknown, this vascular toxicity appears to be dose-related and modified by pre-existing CV disease and other risk factors.
Cortes: ARIAD: Consultancy, Research Funding; Teva: Research Funding; BMS: Consultancy, Research Funding; Sun Pharma: Research Funding; ImmunoGen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding. Nicolini: Incyte Biosciences: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; ARIAD: Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau. Hochhaus: ARIAD: Research Funding; Novartis: Research Funding; Incyte: Research Funding; BMS: Research Funding; MSD: Research Funding; Pfizer: Research Funding. le Coutre: Incyte: Honoraria; Pfizer: Honoraria; BMS: Honoraria; Novartis: Honoraria, Research Funding; ARIAD: Honoraria. Kim: Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Il-Yang: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Pinilla-Ibarz: ARIAD: Consultancy, Honoraria; Pfizer: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau. Chuah: Chiltern: Honoraria; BMS: Honoraria, Other: Travel; Novartis: Honoraria; Avillion: Honoraria. Apperley: Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses , Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding; Sun Pharma: Honoraria; Incyte: Honoraria; Therakos: Honoraria; Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Talpaz: Pfizer Inc: Consultancy, Other: Travel, Research Funding; ARIAD: Other: Travel, Research Funding. DeAngelo: Pfizer Inc.: Consultancy, Honoraria, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; BMS: Consultancy; Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; Glycomimetics: Research Funding; ARIAD: Consultancy, Research Funding; Celgene: Research Funding; Immunogen: Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Shire: Honoraria; Blueprint Medicines: Honoraria, Research Funding; Incyte: Consultancy, Honoraria. Abruzzese: Bristol Myers Squibb: Consultancy; Incyte: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; ARIAD: Consultancy. Rea: Novartis: Consultancy, Honoraria; Pfizer: Honoraria; Incyte: Honoraria; BMS: Consultancy, Honoraria. Mueller: ARIAD: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; IHO GmbH: Equity Ownership. Gambacorti-Passerini: Pfizer: Consultancy, Honoraria, Research Funding; BMS: Consultancy. Castagnetti: Novartis: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Lustgarten: ARIAD: Employment, Equity Ownership. Neumann: Takeda Pharmaceuticals Inc: Employment, Equity Ownership. Clackson: ARIAD: Employment, Equity Ownership. Guilhot: ARIAD: Honoraria. Deininger: BMS: Consultancy, Research Funding; Incyte: Consultancy; Ariad Pharmaceuticals, Bristol Myers Squibb, CTI BioPharma Corp, Gilead, Incyte, Novartis, Pfizer, Celgene, Blue Print, Galena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; ARIAD: Consultancy; Celgene: Research Funding; Gilead: Research Funding. Hughes: Ariad: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Shah: Bristol-Myers Squibb: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding. Kantarjian: Pfizer: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding; Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; ARIAD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal